Funding History & Goals
Awarded Grants
CISNET
is funded through an NCI cooperative agreement (U01) mechanism, which ensures substantive NCI involvement in attaining research goals and catalyzing collaborations both between consortium members and with outside groups seeking collaborations. There are six phases of funding:Current Programs
Previous Programs
| CISNET Incubator 2021-2026 |
CISNET V 2020-2025 |
CISNET IV 2015-2020 |
CISNET III 2010-2015 |
CISNET II 2005-2010 |
CISNET I 2000-2005 |
|
|---|---|---|---|---|---|---|
| Bladder Cancer | ||||||
| Breast Cancer | ||||||
| Cervical Cancer | ||||||
| Colorectal Cancer | ||||||
| Esophageal Cancer | ||||||
| Gastric Cancer | ||||||
| Lung Cancer | ||||||
| Multiple Myeloma | ||||||
| Prostate Cancer | ||||||
| Uterine Cancer |
CISNET Incubator Program for New Cancer Sites (2021-2026)
The CISNET Incubator Program, funded under RFA-CA-20-043, is focused on expanding comparative simulation modeling approaches developed by CISNET to new cancer organ sites beyond the sites that have been previously funded. The Program aims to translate CISNET’s framework of success to cancer sites that are not already part of CISNET and for which there has been nascent/limited population modeling and little to no comparative modeling. Accelerating the development of comparative simulation modeling approaches for the cancer sites funded under this Program (bladder, gastric, multiple myeloma, and uterine) is expected to generate sophisticated, evidence-based decision tools that could inform decisions on the most efficient utilization of existing and emerging technologies and strategies for the control of these cancers.
CISNET V (2020-2025)
CISNET V, funded under RFA-CA-19-054, is focused on the continued generation of sophisticated, evidence-based decision tools to inform international/national/regional/local decisions on the most efficient utilization of existing and emerging technologies and strategies for the control of cancer. These tools are expected to promote:
- Enhanced understanding of the impact of cancer control interventions (screening, treatment, prevention) on current and future trends in incidence and mortality;
- Determination of the most efficient and cost-effective strategies for implementing technologies in the population, based on extrapolated evidence from clinical trials, epidemiologic studies, and observational studies; and
- Determination of which new, emerging technologies are the most promising for scaling up to the population level, based on data from limited-scale studies.
As with the prior two CISNET funding phases, the CISNET V RFA encouraged modeling across the entire cancer control spectrum (i.e., prevention, screening, diagnosis, treatment, surveillance, and end-of-life care). The CISNET V RFA specified nine targeted priority areas:
- Precision Screening and New Screening Technologies;
- Precision Treatment;
- Overdiagnosis and Active Surveillance;
- Decision Aids (Individual and Policy);
- Understanding Screening in Real-World Settings and Determining the Best Routes to Optimize the Processes;
- State, Local, and International Cancer Control Planning;
- Suggesting Optimal Routes to Reduce Health Disparities;
- Methods Development;
- Cancer-Type Specific Opportunities.
In September 2020, five multiple PI grants were awarded, for breast, lung, prostate, colorectal, and cervical cancers.
CISNET IV (2015-2020)
CISNET IV, funded under RFA-CA-14-012, focused on the continued generation of sophisticated, evidence-based decision tools to inform international/national/regional/local decisions on the most efficient utilization of existing and emerging technologies and strategies for the control of cancer. As with CISNET III, the CISNET IV RFA encouraged modeling across the entire cancer control spectrum (i.e., prevention, screening, diagnosis, treatment, surveillance, and end-of-life care). The CISNET IV RFA specified nine targeted priority areas:
- Exploring the evolving potential of stratification based on polygenic risk for cancer screening and genomic tumor profiles for treatment;
- State, local, and international cancer control planning;
- Understanding how screening and treatment work in real-world settings and determining the best routes to optimize the processes;
- Assisting in the development of decision support tools;
- Evaluating natural experiments generated as a result of the Affordable Care Act (ACA);
- Value of information analyses;
- Pan-cancer and pan-disease modeling;
- Suggesting optimal routes to reduce health disparities; and
- Cancer-specific opportunities.
In September 2015, six multiple PI grants were awarded, five of which were previously existing CISNET sites (breast, lung, prostate, colorectal, esophagus), and one new site was added (cervix).
CISNET III (2010-2015)
CISNET III, funded under RFA-CA-09-024, focused on meeting the expanding scientific need for tools which assist in synthesizing emerging evidence in a timely manner due to the extraordinary pace of developments in cancer control technologies, basic science studies investigating molecular and biological determinants of cancer risk, upcoming results from clinical trials, and new health-related data. The CISNET III RFA encouraged modeling across the cancer control spectrum (i.e., prevention, screening, diagnosis, treatment, surveillance, and end-of-life care) and expansion in nine targeted areas of special interest:
- Multi-scale modeling;
- Incorporating genomic and family history risk profiles;
- Upstream modeling, i.e. the determinants of trends in risk factors, screening and treatment;
- Modeling aimed at addressing issues in comparative effectiveness (CER)
- Evaluation of diagnostic tests;
- Optimizing biomarker development strategies;
- Suggesting optimal routes to reducing health disparities;
- Translation of trial results into clinical guidelines and public health policy; and
- Developing interactive policy-level decision making tools.
In the prior rounds of CISNET each modeling group applied and was funded separately. Because by the time of this solicitation the work of the modeling groups were highly interconnected, multiple PI applications were encouraged where each application put forward a suite of complementary models and a coordinated plan of interdisciplinary research. Applicants were expected to propose collaborative, interactive projects involving groups of researchers that would put forward a program of comparative modeling with coverage across the important cancer control issues and relevant specific focus areas for the selected organ site. A coordinating center was built into the application, and one of the PIs is designated as the coordinating center PI. The role of the coordinating center is:
- Formulating, prioritizing, and coordinating work on base case and other questions (including outside requests),
- Negotiating common requests for outside data sources, (3) Consensus building and coordinating critical evaluation of disparate results;,
- Preparing inputs and collecting and processing common outputs for model comparisons,
- Coordinating synthesis papers and group responses, bringing together disparate information to inform policy makers; and
- Organizing conference calls and setting meeting agendas.
In September 2010, five multiple PI grants were awarded, four of which were in the previously existing CISNET cancer sites (breast, lung, prostate, colorectal) and one new site was added (esophagus).
CISNET II (2005-2010)
CISNET II, funded under RFA-CA-05-018, focused on the application of already developed models to study the population impact of existing or emerging cancer control interventions. CISNET II was an opportunity for new applicants who had working models of one of the CISNET cancer sites to apply for funding to join the CISNET consortium as well as for applicants funded under earlier solicitations to be re-funded. CISNET II also provided the opportunity to establish centers that are dedicated to a specific cancer site for coordinating activities such as joint analyses and synthesis papers, collaborations with outside researchers, and group requests for data resources.
The goal of CISNET II was to promote the application and extension of models to answer vital questions related to emerging cancer trends, the impact of interventions on future trends (development phase), and the identification of optimal strategies for reducing the cancer burden (delivery phase).
CISNET I (2000-2005)
CISNET I was funded in two rounds; the first round funded grants on breast, colorectal, and prostate cancers (RFA-CA-99-013), and the second round added lung cancer as well as additional grantees for colorectal and prostate cancers (RFA-CA-02-010). CISNET I provided the support needed to develop applicable mathematical and statistical methodologies and incorporate those methodologies into multi-cohort population models.
CISNET Provides Tools for the Evaluation of the Delivery of Interventions at the Population Level
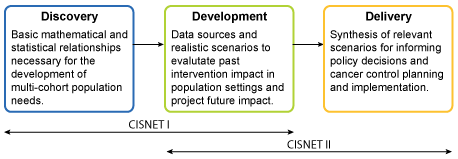
CISNET and the Challenge of Delivery. CISNET I goals focused on the discovery-development phases while CISNET II focused on the development-delivery phases..
During this initial phase, an infrastructure was put into place to facilitate communication among modelers and cross-model comparisons. This discovery phase involved the development of basic mathematical and statistical methodology that is necessary to build multi-cohort population models. Then, during the development phase, these models were validated using available data and applied to the population setting to answer relevant research questions.
CISNET I projects focused on understanding the impact of interventions (e.g., prevention, screening, and treatment) on current population trends in incidence and mortality.