ENGAGE University of Texas MD Anderson Cancer Center
ENGAGE is a comprehensive lung cancer screening framework that provides individualized screening and diagnostic recommendations informed by the personal lung cancer risk and life expectancy of ever-smoked individuals.
Contact: Iakovos Toumazis itoumazis@mdanderson.org
Overview
The individualizEd luNG cAncer screeninG dEcisions (ENGAGE) is a comprehensive lung cancer screening framework that provides individualized screening and diagnostic recommendations informed by the lung cancer risk and life expectancy of ever-smoked individuals. Key features of ENGAGE include: (i) optimization of lung cancer screening at the individual-level; (ii) optimization of the management of screen-detected suspicious pulmonary nodules and recommendation of the optimal diagnostic modality; (iii) development of microsimulation natural history model to simulate the disease progression of pulmonary nodules by sex, histological subtype, and nodule type (solid, part-solid, non-solid); (iv) development of a risk prediction models for lung cancer incidence and competing causes of death; (v) integration of life expectancy will allow to optimize screening’s starting age, stopping age, and screening frequency; (vi) design of adaptive screening schedules by incorporating information collected from previous screening exams and changes in the smoking behavior of individuals.
ENGAGE formulates the decision of whether to screen an individual at a given age as a partially observable Markov decision process (POMDP) to optimize cancer screening decisions. ENGAGE formally assesses the benefits and harms of screening annually, while accounting for the uncertainty stemming from the unobservable health state of individuals, unknown future health trajectory, and imperfect information collected from screening modalities.1 The ENGAGE screening decisions (i.e., Screen or No Screen) are based on a real-time assessment of an individual’s health state, lung cancer risk, and life expectancy.1 ENGAGE is validated on the Surveillance, Epidemiology, and End Results (SEER) and National Lung Screening Trial (NLST) and has been shown to be more effective and efficient that the existing guidelines on lung cancer screening.1,2 ENGAGE considers both smoking-related (e.g., smoking status, smoking intensity, years since smoking cessation) and non-smoking-related risk factors (age, race and ethnicity, BMI, comorbidities, and personal history of cancer, among others) to provide personalized, analytically optimal screening schedules that adapt to past screening findings and changes in modifiable risk factors.
ENGAGE is informed by risk models that assess the personal risk of individuals for developing lung cancer, death from causes other than lung cancer, and risk of malignancy for screen-detected pulmonary nodules. Moreover, smoking-state transition probabilities are estimated using data from the Population Assessment of Tobacco and Health (PATH) Study and the smoking history generator (SHG) which is a well validated microsimulation model developed within Cancer Intervention and Surveillance Modeling Network (CISNET).3-5 The lung cancer incidence and mortality risks from competing causes of death are estimated using risk prediction models developed using data from the PLCO trial, which have been validated on the NLST. There are three versions of the PLCOENGAGE2025 model differing by the number of racial and ethnic groups considered in the model (6-race, 3-race, and no-race versions). ENGAGE is flexible and can incorporate alternative risk prediction models (e.g., Bach, PLCOm2012).6,7 Similarly, we developed a risk prediction model that estimates the risk of death from causes other than lung cancer using data from the PLCO trial. The developed ENGAGE risk models incorporate both smoking-related (smoking intensity, smoking duration, smoking status, and years since smoking cessation) and non-smoking-related covariates (race and ethnicity, sex, age, body-mass index, education level, chronic obstructive pulmonary disease, personal history of cancer, family history of LC, and comorbidities such as diabetes, cardiovascular disease, stroke, and hypertension). For assessing the risk of malignancy of screen-detected pulmonary nodules, ENGAGE uses the Brock model and makes personalized recommendations on the best available intervention among serial low-dose CT, PET/CT, biopsy, biomarker testing, and do nothing.
To estimate transitions between cancer states, ENGAGE uses a newly developed natural history model (NHM) that simulates the natural progression of lung cancer in the absence of any intervention by sex, histological subtype (small cell carcinoma, squamous cell carcinoma, adenocarcinoma, and other non-small cell carcinoma), and nodule type (solid, part solid, and non-solid). We modeled the natural progression of lung cancer as a discrete-time Markov chain. The states of the NHM describe the health status of the patient’s cancer staging in terms of the AJCC staging (with ongoing efforts to expand to the TNM staging system). All individuals start from the “healthy” state at age 18 and transition between states based on transition probabilities until death or age 100, whichever occurs first. State transitions upon the onset of lung cancer to death depend on the size of the tumor, which is modeled as a Gompertz function. The model was calibrated to data from the Surveillance, Epidemiology, and End Results (SEER) registry with 17 regions (2000-2021) in terms of survival time after diagnosis, stage distribution at diagnosis, tumor size distribution at diagnosis, and age distribution at diagnosis by stage.
ENGAGE dynamically assesses the probability that an individual will be screen-detected with lung cancer if they undergo screening at that specific moment given that person's risk factors and screening histories; hereon called ENGAGE-derived lung cancer prevalence. ENGAGE assesses the impact of screening by overlaying screening on the NHM. The first screening decision is made at age 50 years and it is based on the personal lung cancer risk of the individual estimated by the risk model and the tradeoffs between the expected benefits of detecting lung cancer earlier and the potential harms of screening (anxiety, cost, false-positives, and overdiagnosis, among others). Once the optimal action at a given time t, denoted αt, is taken (i.e., Screen or No Screen), an observation, ot, is realized (Normal Screen, Abnormal Screen, Presence/Absence of Clinical Symptoms). Before making the next decision, ENGAGE updates the risk factors of the individual, assimilates newly collected information from past findings, and calculates the ENGAGE-derived prevalence using Bayesian updating [Equation (1)]. The updated ENGAGE-derived prevalence is then used to inform the next decision, and the process repeats itself until lung cancer diagnosis, death, or age 100 years, whichever occurs first.

for all, 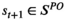 where s denotes the individual's health state,
where s denotes the individual's health state, 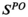 represents the set of unobservable health states, and b represents the ENGAGE-derived lung cancer prevalence.
represents the set of unobservable health states, and b represents the ENGAGE-derived lung cancer prevalence.
Upon the detection of a pulmonary nodule, ENGAGE assesses the risk of malignancy of that specific nodule using the Brock model which informs a second POMDP model that provides analytically optimal recommendations on the diagnostic modality to be used. Available interventions include serial low-dose CT, PET/CT, biopsy, biomarker testing, as well as the do-nothing option. Decisions are being made monthly, and the objective of the POMDP model is to identify the optimal action and the timing such that the expected quality-adjusted life years of an individual are maximized.
The ENGAGE framework facilitates individualized, dynamic risk-based screening and diagnostic recommendations, that informs screening guidelines by identifying individuals who are most likely to benefit from screening and ensuring that suspicious screening findings are being timely managed with the optimal intervention.
References
- Toumazis I, Alagoz O, Leung A, Plevritis SK. A risk‐based framework for assessing real‐time lung cancer screening eligibility that incorporates life expectancy and past screening findings. Cancer. 2021 Dec 1;127(23):4432-46.
- Hemmati M, Ishizawa S, Meza R, Ostrin E, Hanash SM, Antonoff M, Schaefer AJ, Tammemägi MC, Toumazis I. Benchmarking lung cancer screening programmes with adaptive screening frequency against the optimal screening schedules derived from the ENGAGE framework: a comparative microsimulation study. EClinicalMedicine. 2024 Jul 28;74:102743. doi: 10.1016/j.eclinm.2024.102743. PMID: 39764179; PMCID: PMC11701438.
- Hyland A, Ambrose BK, Conway KP, Borek N, Lambert E, Carusi C, Taylor K, Crosse S, Fong GT, Cummings KM, Abrams D, Pierce JP, Sargent J, Messer K, Bansal-Travers M, Niaura R, Vallone D, Hammond D, Hilmi N, Kwan J, Piesse A, Kalton G, Lohr S, Pharris-Ciurej N, Castleman V, Green VR, Tessman G, Kaufman A, Lawrence C, van Bemmel DM, Kimmel HL, Blount B, Yang L, O'Brien B, Tworek C, Alberding D, Hull LC, Cheng YC, Maklan D, Backinger CL, Compton WM. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 2017 Jul;26(4):371-378. doi: 10.1136/tobaccocontrol-2016-052934. Epub 2016 Aug 8. PMID: 27507901; PMCID: PMC5299069.
- Jeon J, Holford TR, Levy DT, Feuer EJ, Cao P, Tam J, Clarke L, Clarke J, Kong CY, Meza R. Smoking and lung cancer mortality in the United States from 2015 to 2065: a comparative modeling approach. Annals of internal medicine. 2018 Nov 20;169(10):684-93.
- Holford TR, Clark L. Chapter 4: Development of the counterfactual smoking histories used to assess the effects of tobacco control. Risk Analysis: An International Journal. 2012 Aug;32:S39-50.
- Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, Hsieh LJ, Begg CB. Variations in lung cancer risk among smokers. Journal of the National Cancer Institute. 2003 Mar 19;95(6):470-8.
- Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, Commins J, Berg CD. Selection criteria for lung-cancer screening. New England Journal of Medicine. 2013 Feb 21;368(8):728-36.
Bladder models
- Kystis (Brown) Brown
- COBRAS (Ottawa) Ottawa
- SCOUT (NYU) NYU
Bladder Model Comparison Grid (PDF, 145 KB)
See all Comparison Grids & Profiles (Includes historical versions)
Lung models
- BCCRI-LunCan (BCCRI)
- BCCRI-Smoking (BCCRI)
- LCOS (Stanford)
- LCPM (MGH)
- MISCAN-Lung (Erasmus)
- SimSmoke (Georgetown)
- Smoking-Lung Cancer (Georgetown)
- MULU (Mount Sinai)
- ENGAGE (MDACC)
- YLCM (Yale)
- OncoSim-Lung (CPAC-StatCan)
- LMO (FHCC) (Historical)
Lung Model Comparison Grid (PDF, 161 KB)
See all Comparison Grids & Profiles (Includes historical versions)